Products
View our Buoyant Microbubble Cell Separation Products
Cell Separation Products
Buoyant Microbubble Cell Separation Products
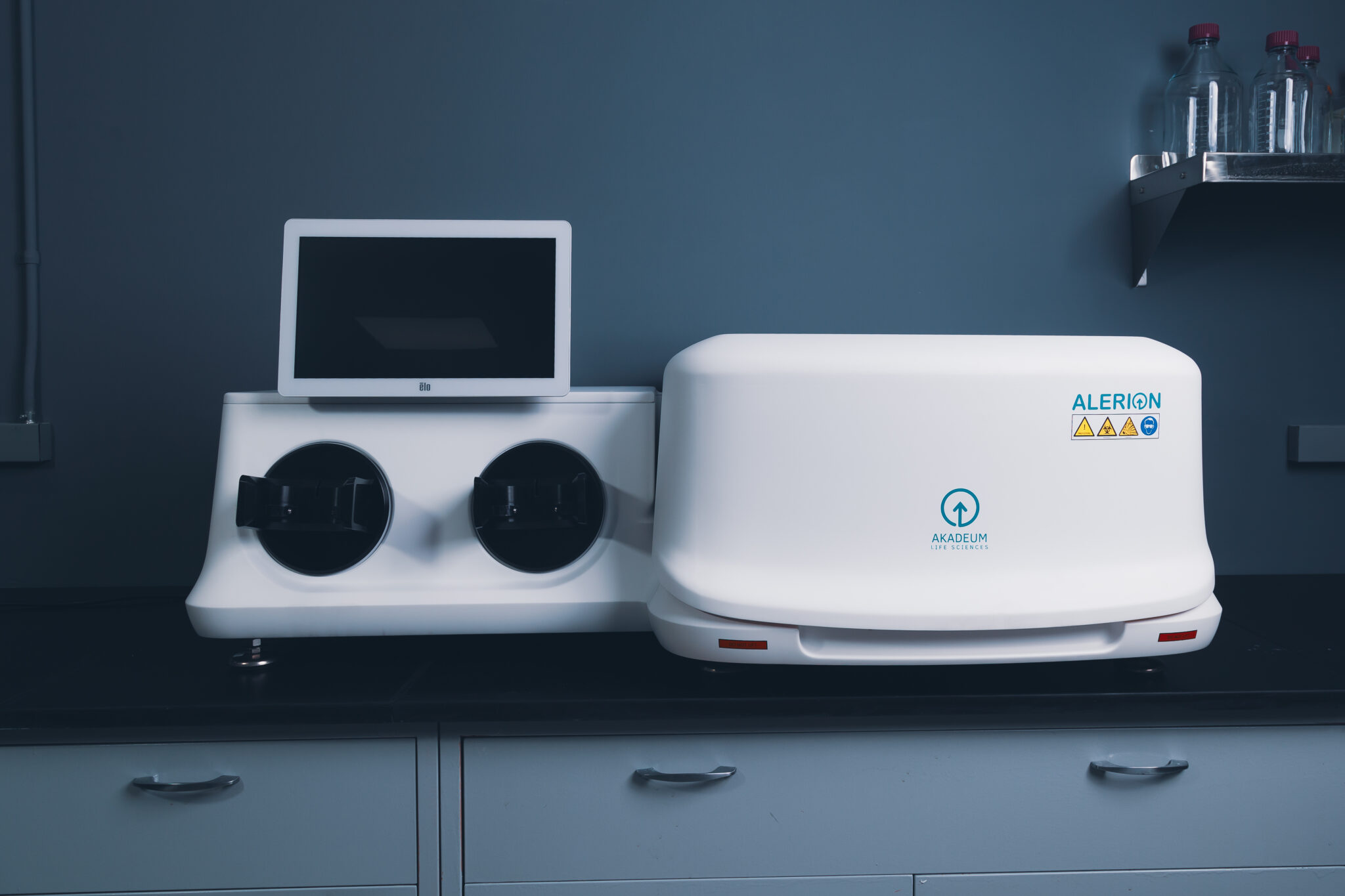
Akadeum Life Sciences® has developed a next-generation platform that solves long-standing problems across cell therapy and other research, diagnostic, and therapeutic markets, including the first at-scale negative selection kit. Kits are available for cell therapy, purification, culture cleanup, and small-scale immunology research.
GMP-grade and Clinical-Ready products are available for the cell therapy product line, enabling research through the commercialization of cell therapeutics.