Living Cells can React to Magnetic Signals
Updated on Aug 4, 2023 Share
Researchers and scientists around the world have long theorized that the inherent directional systems in various animals (including migratory animals like birds, butterflies, whales, and possibly even humans) could have a magnetic component – that internal navigational impulses are related in some way to Earth’s magnetic field. While this has been a long-standing theory, the underlying cellular mechanisms that support the ability of an organism to detect magnetic field to perceive direction, altitude, or location have remained undefined – until now.
Recently, a research group from University of Tokyo has identified the cellular response to magnetic signals at a chemical level. The team have characterized the first observations of biological magnetoreception – the cellular response of living, untouched cells to magnetic signals in real-time. This is a novel and groundbreaking observation that could lead to a number of answers regarding the underlying biological mechanisms that drive migratory patterns, the impact of low-level electromagnetic fields on human health, and the implications of using magnetic forces for sample preparation and cell separation in ongoing research.
Cryptochromes and Magnetic Fields
Scientific knowledge has theorized that the underlying mechanism powering magnetoreception is related to the influence of magnetic fields on the electrons of certain molecules. When a magnetic field interacts with these molecules, it can modify the electron’s spin state which can then lead to downstream impacts like regulating the speed at which certain chemical reactions occur.
One such impact relates to cryptochromes, a class of flavoproteins that are known to exist in both plants and animals. Cryptochromes are regulated by two genes, Cry1 and Cry2 that are responsible for the related proteins CRY1 and CRY2. Cryptochrome is famously sensitive to blue light and is involved in innate biological processes like circadian rhythm and magnetoreception.
In response to different factors, cryptochrome forms a radical pair with correlated spins. The single electrons of each member of the pair can exist in two different spin states – if they are spinning in parallel, downstream chemical reactions are slowed down whereas if they are spinning opposite of one another reactions can occur more rapidly. It has long been postulated that magnetic forces can impact these spin states, which would then directly influence subsequent chemical reactions that involve cryptochrome radical pairs.
In recent decades, the presence of cryptochromes have been identified and characterized through in vitro observations, and it has been demonstrated that genetic interference with cryptochromes can remove the ability for insects like fruit flies and cockroaches to use geomagnetic navigation. Still, the direct chemical changes occurring at the cellular level, in a living cell, in response to magnetic signals, had not been directly observed or defined until now.
Shedding Light on the Underlying Chemical Changes
To investigate the direct effect of magnetic fields on living cells, a research team from University of Tokyo looked specifically at HeLa cells. The results of their research have been recently published in PNAS. HeLa cells are commonly used for a wide range of research applications and have become notorious in recent years as they were initially obtained from cancer patient Henrietta Lacks without her consent and subsequently cultured in vitro to produce the prolific immortal cell line. HeLa cells contain flavin molecules, a subset of cryptochromes. These flavins will fluoresce (glow) or produce radical pairs when exposed to blue light.
Because flavins will either fluoresce or produce radical pairs, there is an inherent competition between the two pathways that can be measured – meaning that the amount of fluorescence observed will depend on how quickly the radical pairs react. Since the speed of chemical reactions is influenced by the presence of parallel or anti-parallel spin patterns, and these spin patterns are thought to be influenced by magnetic forces, the researchers can then monitor changes in fluorescence in the presence or absence of an artificial magnetic field to see whether the chemical changes that have, to this point, been largely theoretical could be specifically characterized and observed.
The team irradiated the HeLa cells with blue light to stimulate fluorescence, as blue light is thought to play a role in the development of radical pairs. Because autofluorescence can be common in cells, the team isolated flavin fluorescence specifically using lasers to monitor wavelength. They also controlled for environmental factors (such as the heat that can be generated by the equipment used) to ensure that the application of the artificial magnetic field was the only variable in the cells’ environment.
Over a period of 40 seconds, a magnetic field with roughly equivalent power to a common refrigerator magnet was swept across the irradiated cells every four seconds and changes to the intensity of the light being emitted were recorded. The researchers found that cryptochrome levels would increase or decrease based on how near or far the magnet was placed in relation to the cells. When the magnet came into close proximity, the glow visibly diminished, indicating chemical changes at the cellular level in response to the interference of magnetic force. On average, fluorescence was observed to have decreased by ~3.5% in the presence of the magnetic field and returned to normal when the magnetic force was removed. The cells themselves were not modified or engineered; the observed changes appear to be the result of chemical activity happening at the cellular level in response to the presence of magnetic forces.
Magnetic Activated Cell Sorting (MACS)
Sample preparation is a critical step in nearly any research activity that begins with a biological sample. Before further processing can begin, the sample first needs to be optimized to isolate the specific components of interest – this can include proteins, DNA/RNA, or specific cell types. One of the most common methods for sample preparation uses magnetic beads – the beads are mixed into the sample, where they attach to the target of interest. The sample is then processed through a magnetic column. The column will then hold the captured cells to the side, while the remainder of the sample is poured off.
There are several disadvantages to this process, especially when working with rare or delicate cell types. It can be incredibly difficult to obtain a large enough population of cells of interest from a biological sample – various buffers, chemicals, and exposure to magnetic forces can all play a part in decreased cell count and viability. Using magnetic bead separation also requires the use of specialized equipment, including the use of rare earth magnets, and consumables such as magnetic columns.
As research continues to build upon these new discoveries, it seems increasingly likely that it would be beneficial for researchers to avoid exposing target cells to unnecessary magnetic forces as it remains unknown exactly what impact this could have on the viability, characterization, activation, and study of different cell types.
Buoyancy Activated Cell Sorting (BACS™)
Novel technologies, like buoyancy-activated cell sorting, can provide researchers with an alternative to processing samples with magnetics. BACS™ uses functionalized microbubbles that are mixed into the sample, but instead of requiring rare earth magnets and the application of magnetic fields, the bubbles are buoyant and simply float to the surface of the vial. The separation happens directly in the sample container (without the need for additional specialized equipment) and can be easily scaled up in number or volume to accommodate the specific research application.
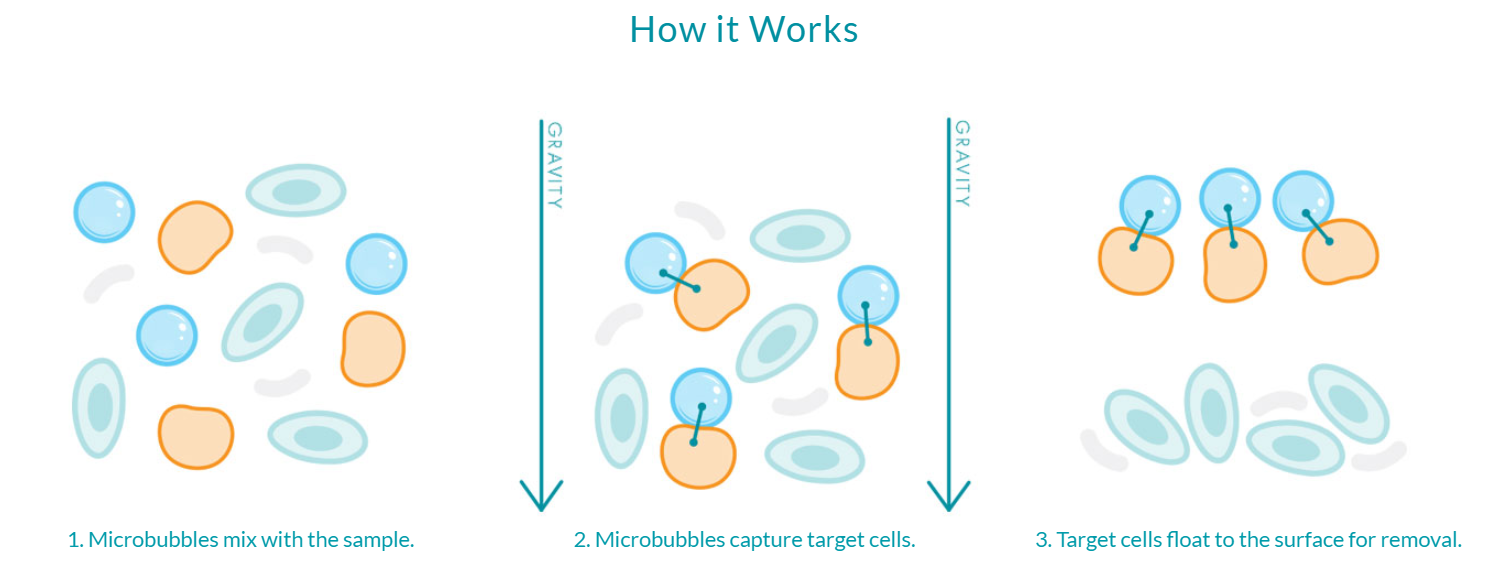
Because BACS™ is powered by microbubbles, the only force acting upon the sample is one that already exists – gravity. The elegance of the microbubble solution is that it is simple and powerful while being incredibly gentle on rare or delicate cells of interest. Microbubbles can eliminate the need to expose cells to harsh chemicals or external forces like rare earth magnets, ultimately resulting in a highly-enriched, viable population of cells for downstream processing and analysis.
Where the research is heading next
The Earth’s natural magnetic field, while variable depending on location, is about 500 times weaker on average than the magnetic forces tested in the study. This is especially interesting for building upon the Tokyo group’s research, as the low field effect could play a critical role in biological magnetoreception and other important biological processes. Strong magnetic fields, like those used in magnetic bead separation or common refrigerator magnets, can make it more difficult for radical pairs to switch how they spin in relation to one another. This implies that a strong magnetic field has a direct impact on this essential behavior within cells. Weaker magnetic fields – like the natural magnetic field of the Earth – can have the opposite effect, making it easier for radical pairs to make this switch. Understanding the impact of Earth’s weaker magnetic field could further scientific knowledge of how cells interact with the natural magnetism of the Earth on the molecular level.
The Tokyo researchers are looking to next investigate the impact of magnetic fields on other types of cells, as well as studying the role of cellular health and environment on the observed responses. Of additional interest is potential magnetic receptors, including the cryptochromes inside cells. Specialized and highly sensitive equipment will be of critical importance for enabling more detailed cellular analysis to analyze the possible significance of new developments and discoveries, especially in investigations looking to characterize the magnetic response to signaling pathways within the cell.
There is still much to learn – which is, undoubtedly, one of the most exciting things about scientific research. Every new discovery brings with it the potential for deeper understanding of the natural world and the possibility of positively impacting the advancement of human health as well as the health of the environment around us.
Further Reading and References:
- Noboru Ikeya, Jonathan R. Woodward. Cellular autofluorescence is magnetic field sensitive. Proceedings of the National Academy of Sciences, 2021; 118 (3): e2018043118 DOI: 10.1073/pnas.2018043118
- University of Tokyo. “Magnets dim natural glow of human cells, may shed light on how animals migrate: First direct observation of magnetic field affecting autofluorescence of flavins in living cells.” ScienceDaily. ScienceDaily, 5 January 2021. www.sciencedaily.com/releases/2021/01/210105104832.htm>.
- Rodgers CT, Hore PJ. Chemical magnetoreception in birds: the radical pair mechanism. Proceedings of the National Academy of Sciences, 2009. 106 (2): 353–60. Bibcode:2009PNAS..106..353R. doi:10.1073/pnas.0711968106. PMC 2626707. PMID 19129499.
- Biskup T, Schleicher E, Okafuji A, Link G, Hitomi K, Getzoff ED, Weber S. Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angewandte Chemie, 2009. 48 (2): 404–7. doi:10.1002/anie.200803102. PMC 4329312. PMID 19058271.
- Chandler D, Ilia Solov’yov I, Schulten K. Cryptochrome and Magnetic Sensing. Beckman Institute for Advanced Science and Technology, University of Illinois Urbana–Champaign.
Learn more about Microbubbles
Buoyancy-Activated Cell Sorting (BACS™), Akadeum’s patented approach to separation, offers a novel way to isolate the target of interest (cells, nucleic acids, proteins, chemicals, etc) using science so simple, it floats! We functionalize tiny floating particles that we call “microbubbles” and develop the protocols and devices to put our microbubbles to work. Our technology combines the specificity of affinity molecules such as antibodies with the power of gravity in a workflow that’s fast, easy, and effective while being exceptionally gentle on delicate targets, like rare immune cells of low abundance.


