Optimizing Sample Prep for Flow Cytometry
Updated on Sep 27, 2024 Share
Inadequate sample preparation is harmful and costly.
Akadeum has risen to meet your needs.
Akadeum’s next-generation technology overcomes long-standing headaches with traditional sample preparation like magnetic bead-based separation. Stop losing your precious samples to magnetic processes and experience the benefits of a microbubble workflow.
Sample Preparation Methodology
Depending on the sample being processed and its biological makeup, sample preparation needs can vary widely. From fast and easy cleanup of residual red blood cells to more specific sample preparation to isolate delicate cells of low abundance, it is critical that the sample preparation method being used delivers high-quality cells for downstream use, leaving target cells pure and unaltered by harsh retrieval techniques.
Current technologies have failed to meet the needs of evolving scientific research and industries – and that shortcoming has a negative impact on everything from healthcare costs to research results.
Inadequate sample preparation is harmful and costly. Most biological workflows require an essential sample preparation step in order to isolate the target of interest prior to downstream processing (like flow cytometry or FACS). This step is absolutely critical prior to flow cytometry or FACS, as this ensures that the sample being processed contains as little background noise as possible. Effective sample preparation reduces sort time, increases the quality of results, and delivers a higher population of viable samples for downstream processing and analysis.
Microbubbles for Sample Preparation
Buoyancy-activated cell sorting (BACS™), Akadeum’s patented approach to separation, offers a novel way to isolate the target of interest using science so simple, it floats! Tiny, floating particles called microbubbles are specifically functionalized for any number of applications, including sample preparation for flow cytometry. The microbubble technology combines the specificity of affinity molecules such as antibodies with the power of gravity in a workflow that’s fast, easy, and effective while being exceptionally gentle on delicate targets, like immune cells of low abundance.
This buoyant approach to separation eliminates many of the existing technical hurdles that are limiting improvements or effectiveness of processes today.
Unlike with magnetic bead-based separation, microbubbles do not have the same volume and equipment restrictions. The microbubbles are gently mixed into the sample where they engage with the targets before isolating those targets through floatation-based separation. Instead of technology that traditionally requires additional equipment like magnets and columns to accomplish this cell separation, microbubbles allow for single-container processing that enables cells self-separation.
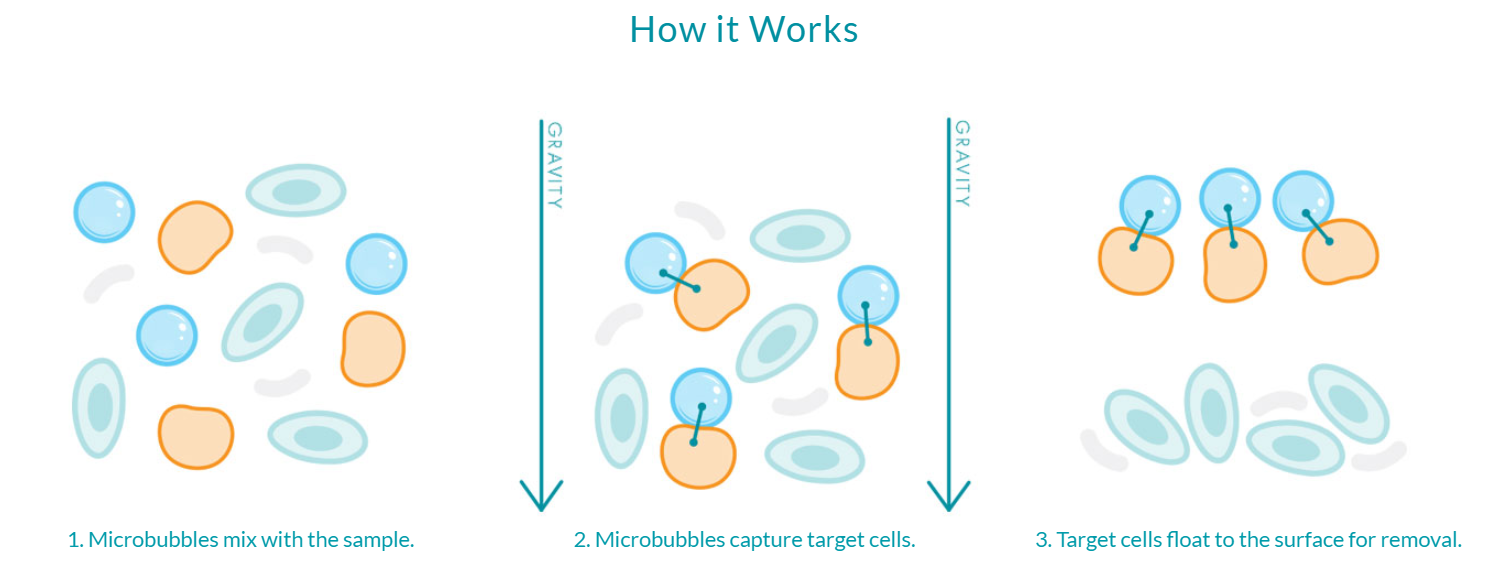
Figure 1: Microbubbles are gently mixed into the sample where they bind to their targets, then isolate those targets through floatation-based separation. This fast and easy workflow can take place directly in the sample container and requires no additional equipment to perform.
With the microbubble workflow, enrichment is performed directly in the sample container, eliminating the need for harsh chemicals, external magnetic forces, and the use of rare earth magnets. Microbubbles harness the power of gravity for separation, maintaining cell health and physiology during enrichment and resulting in an incredibly pure population of rare and delicate cells for downstream applications like flow cytometry.
- Shop Human RBC Depletion Microbubbles
- Shop B Cell Isolation Microbubbles
- Shop T Cell Isolation Microbubbles
- Shop all of Akadeum’s Microbubble Kits
Microbubbles in Action: Removal of Residual RBC Contamination Prior to Flow Sorting
The presence of a high percentage of RBC contamination can impede subsequent cell sorting or other single cell isolation instrumentation. Residual RBCs can extend sort times and occupy wells that are needed for analysis of the relevant cells. While there are various approaches to removing red blood cells from the sample, including lysis buffers like Ammonium Chloride Potassium (ACK), there are concerns about the viability of certain cell types in response to these types of buffers. Additionally, the impact of cellular debris is not well characterized (Sartor et al).
To demonstrate the benefits of using microbubbles for red blood cell (RBC) depletion, 3 unique samples of human peripheral blood mononuclear cells (PBMCs) were prepared with a high level of RBC contamination of ~50%, and each was then divided into two equal samples. The first sample was left untreated. The second sample was depleted of RBCs using Akadeum’s Human Red Blood Cell Depletion Microbubbles. Following depletion, the microbubble fractions demonstrated an average RBC depletion of >95% as compared to the untreated fractions (Figure 2, below).
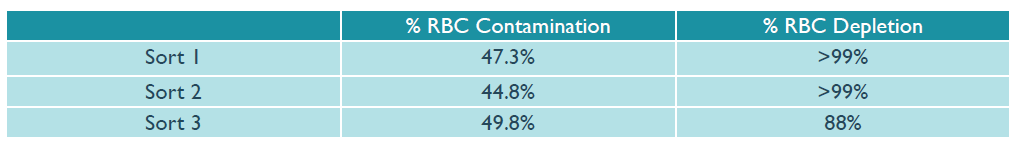
Figure 2: Results of treating three individual samples of RBC-contaminated PBMCs with microbubbles prior to performing the sort.
The two samples were then sorted to collect CD19+ B cells. Depletion of RBCs resulted in a marked increase in the concentration of leukocytes (Figure 3). The samples processed using Akadeum’s Human Red Blood Cell Depletion Microbubbles (Figure 3B and 3D) demonstrated a higher percentage of total lymphocytes and more defined populations than the untreated samples (Figure 3A and 3C).
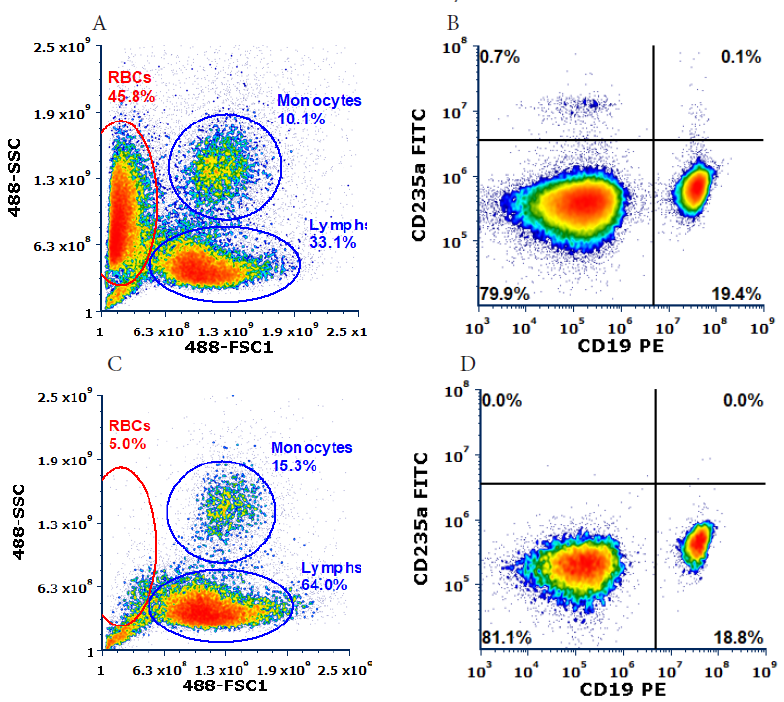
Figure 3: Cell population distributions for the untreated (A&B) and microbubble-depleted (C&D) PBMC samples. B&D are gated on corresponding Lymphs gate (A&C, respectively).
The sorts were performed at approximately 10,000 events per second, and the time to obtain 250,000 CD19+ B cells was recorded. The results of the untreated and microbubble-depleted samples are compared in Figure 4, below.
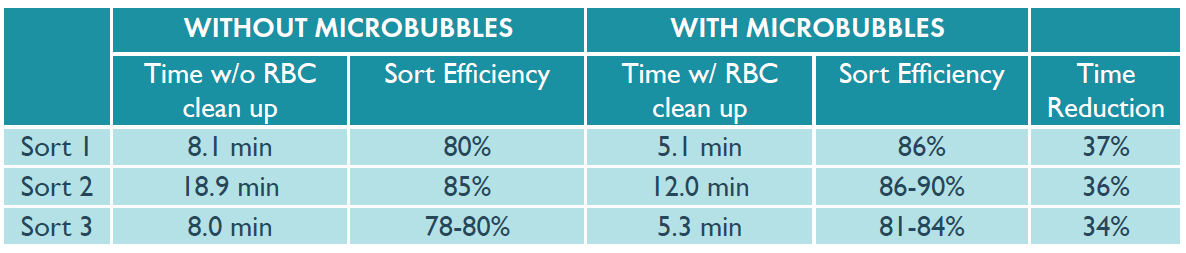
Figure 4: Time required to sort 250,000 CD19+ B cells. Samples were sorted on Beckman Coulter MoFlo Astrios. MoFlo is a trademark of Beckman Coulter, Inc.
Using Akadeum’s Human Red Blood Cell Depletion Microbubbles to remove RBC contamination from the PBMC samples prior to flow cytometry reduced the sort time by an average of 36%.
Additionally, using microbubbles to remove RBC contamination prior to processing increased sort efficiency by up to 6%, as there were fewer events in the stream which reduced the occurrence of RBCs in neighboring droplets leading to aborts.
This data demonstrates that Akadeum’s Human Red Blood Cell Depletion Microbubbles are a simple, effective way to remove unwanted RBCs from samples, which can reduce the sorting time to obtain highly purified cells. With the microbubble workflow, RBC depletion can be achieved in 10 minutes without the need for additional equipment. During longer sorts, regardless of the type of cells being collected, sample preparation with microbubbles can reduce sort times by hours – which not only saves time and resources, but could also improve the quality and viability of the sorted cells.
- Learn More about Microbubbles for RBC Depletion
- Download the RBC Depletion in Human PBMC Samples App Note
- Shop Human RBC Depletion Microbubbles
Microbubbles in Action: Quick and Gentle Isolation of Unique Cell Populations
To ensure high-quality results from downstream applications like flow cytometry and single-cell sequencing, it is imperative that the sample be pure and unaltered by harsh processing techniques. Rare or limited cell populations can be especially challenging to study and often require an additional enrichment step to ensure that enough cells of interest are analyzed to provide meaningful data. While FACS has been the gold standard of cell separation methods, it can be time consuming when isolating cell populations of low abundance. Furthermore, a long sorting step can lead to a significant amount of cell death by the time enough cells are collected for downstream applications. There is a strong need for efficient, rapid, and gentle sample preparation methods to keep up with advancements in technology for assessing numerous aspects of cellular and molecular biology.
Regulatory T cells (Tregs) represent a rare and important cell population in the immune system. They make up 4-10% of the total CD4+ T cell population, and only 1-5% of all PBMCs. A crucial step in investigating Tregs is isolating a large enough population of cells for downstream analysis using separation methods that do not damage the cells or alter their biology.
Because current isolation techniques like magnetic bead-based separation can be time consuming, expensive, and induce stress on cells, development of a gentle and efficient method for obtaining a high purity of a small target cell population for downstream analysis is critical. Using microbubbles for sample preparation ahead of flow cytometry can successfully isolate cells of low abundance like Tregs, while saving time and maintaining high viability, using Akadeum’s Human CD4+ T Cell Isolation Kit. Akadeum’s Human CD4+ T Cell Isolation Kit combines streptavidin-coated microbubbles and a biotinylated antibody cocktail to selectively label, capture, and remove unwanted cell populations.
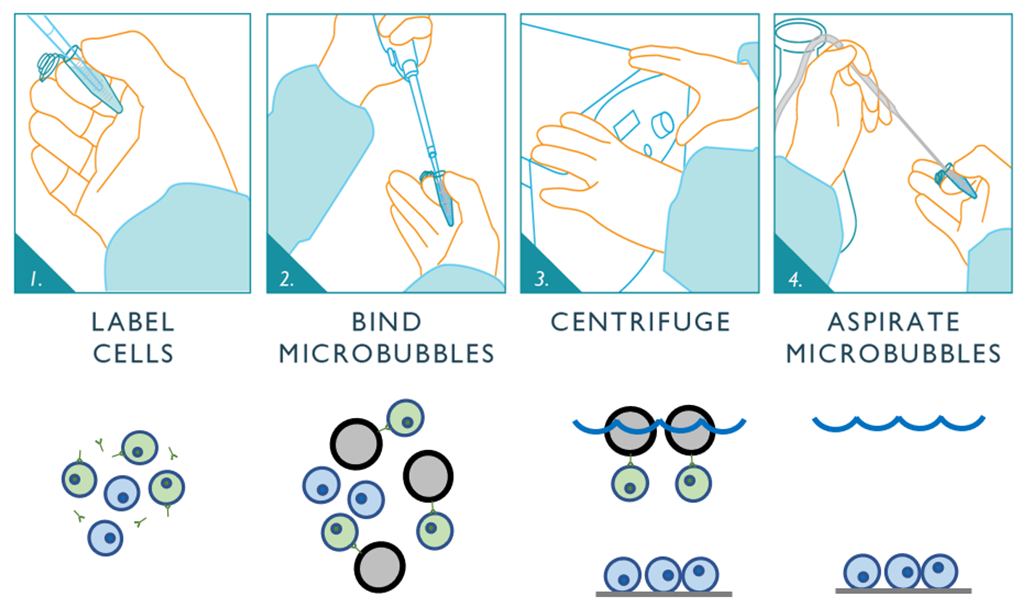
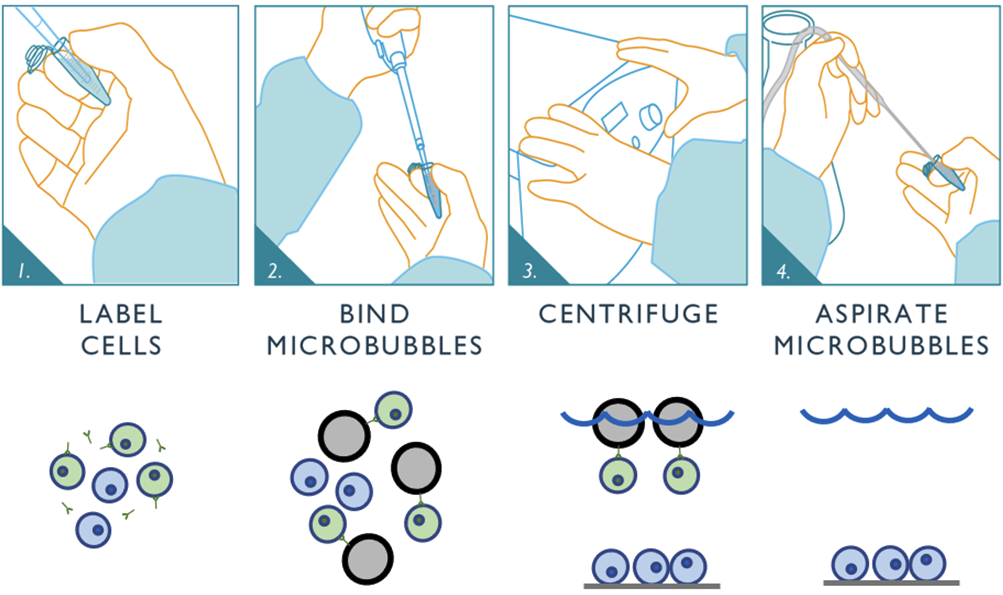
Figure 5: Schematic depicting the negative selection workflow for Akadeum’s Human CD4+ T Cell Isolation Kit. First, the unwanted cells are labeled using Akadeum’s custom, biotinylated antibody cocktail. Next, the streptavidin-coated microbubbles are mixed into the sample where they bind to the labeled cells. The bubble-bound cells are floated to the top of the sample container while the untouched CD4+ T cells of interest remain behind. Vacuum aspiration removes the microbubbles, unwanted cells, and supernatant leaving the highly-enriched, untouched population of CD4+ T cells behind and ready for downstream use.
This rapid, gentle, and effective protocol considerably reduces sort times, thereby lowering time and expense required while improving cell viability (Figure 6).
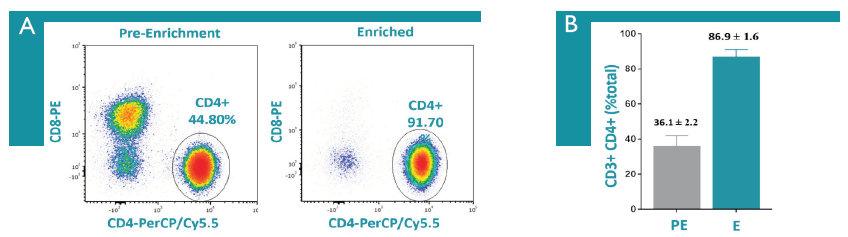
Figure 6: Isolation of human CD4+ T cells from PBMCs was performed, cells were labeled as indicated, and cells were analyzed on a CytoFLEX flow cytometer. A) Flow plots showing an example before and after CD4+ T cell enrichment. B) Before and after CD4+ T cell enrichment results from one-day old PBMCs (n=7). Dead cells and debris were excluded from all analysis.
Using Akadeum’s quick and easy CD4+ T Cell enrichment microbubbles before proceeding with Treg isolation using NanoCellect’s WOLF sorter leads to a significant reduction in overall time required to obtain a sufficient number of highly pure and viable cells that are suitable for downstream analysis. The combined technologies reduce enrichment times 15-fold without sacrificing purity.
- Download the “Quick and Gentle Isolation of Unique Cell Populations” App Note
- Shop Human CD4+ T Cell Microbubbles
Microbubbles for fast, easy, and exceptionally gentle sample preparation ahead of flow cytometry applications
Akadeum’s next-generation technology overcomes long-standing headaches with traditional sample preparation like magnetic bead-based separation. Akadeum’s microbubbles are equipment-free and can enable both low-volume and large-volume separations. Our sensitive and specific microbubbles enable effective capture of targets of low abundance while eliminating issues with non-specific binding.
Critically, the microbubble workflow is exceptionally gentle, which is especially valuable when enriching for delicate targets that can be damaged or lost using older technology like magnetics. With microbubbles, throughput is not limited by access to expensive, specialized equipment which enables sample preparation that can be done at scale.
Microbubbles deliver:
- Increased throughput
- Improved scalability
- Higher sensitivity
- Greater recovery of rare cells
- Faster, easier workflow
- Exceptionally gentle processing on delicate samples
Stop losing your precious samples to magnetic processes and experience the benefits of a microbubble workflow.
At Akadeum Life Sciences, we are committed to improving science through better separation technology. We have a team of scientists who are available to answer any questions you may have – we’d love to hear more about your work and help you develop a workflow that takes advantage of our microbubble technology. Let’s start a conversation!
References:
- Brown M, Wittwer C. Flow Cytometry: Principles and Clinical Applications in Hematology. Clinical Chemistry. 2000 Aug;46(8):1221-1229. doi: 10.1093/clinchem/46.8.1221
- Novo D, Wood J. Flow cytometry histograms: transformations, resolution, and display. Cytometry A. 2008 Aug;73(8):685-92. doi: 10.1002/cyto.a.20592. PMID: 18615596.
- Ormerod M.G., Imrie P.R. (1990) Flow Cytometry. In: Walker J.M., Pollard J.W., Walker J.M. (eds) Animal Cell Culture. Methods in Molecular Biology, vol 5. Humana Press. doi: 10.1385/0-89603-150-0:543
- Sartor, M., Antonenas, V., Garvin, F., Webb, M., and Bradstock, K.F., Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Transplantation 2005; 36: 199-204.