The Role of B Cells and T Cells in COVID-19 Immune Response & T Cell Immunity in COVID-19 Patients
Updated on May 16, 2025 Share
Recent developments in COVID-19 immunology research look at the role T cells and B cells play in immune response to infection with SARS-CoV-2.
The global scientific community has made great strides in characterizing the immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19). There are several studies that have been published in recent months that take a closer look at how B cells and T cells are related to progression of disease. Studies like these are critical for better characterizing the immune system’s response to COVID-19 and our increased understanding will aid in the development of better therapies and vaccines to end the ongoing pandemic.
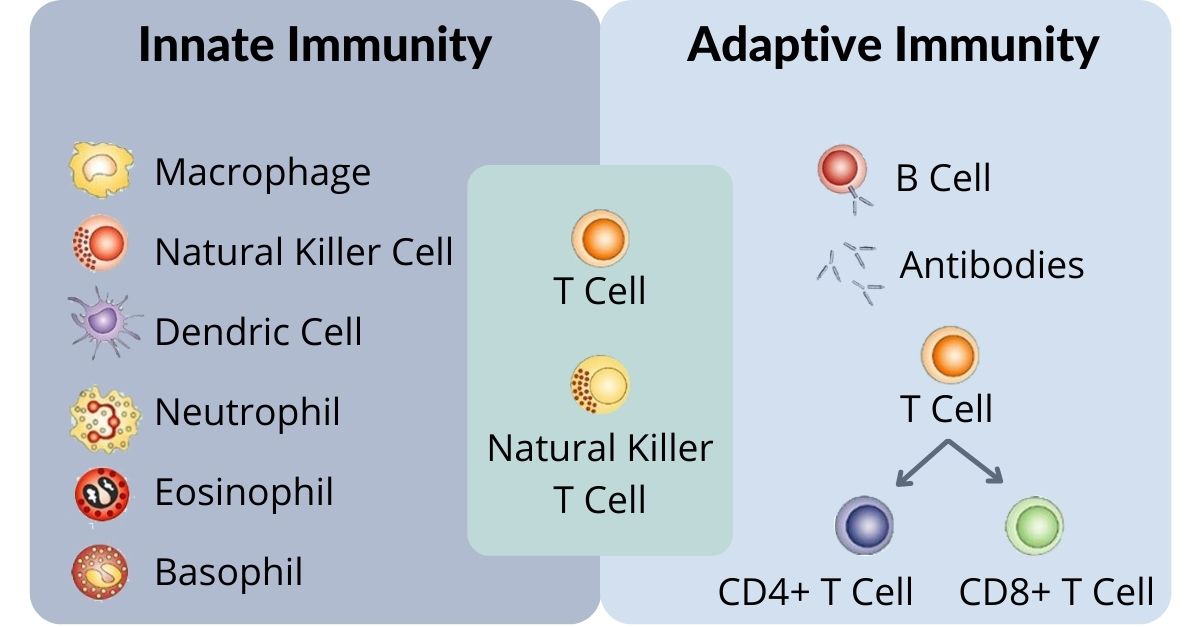
Broadly speaking, the immune system tends to react to viral infection in a relatively predictable way. Serving as the front line of defense is the innate immune system, which rapidly responds in a consistent and broadly-specific manner. The innate immune system consists of macrophages, dendritic cells, monocytes, neutrophils, basophils, eosinophils, natural killer (NK) cells, and mast cells which continuously patrol the body looking for common pathogen-associated molecular patterns (PAMPs). When PAMPs are recognized, a number of responses are triggered. These responses include releasing cytokines and chemokines to alert other immune cells to the discovery. Once recruited, macrophages and dendritic cells phagocytize invading pathogens and display antigens from the digested pathogens on their cell surfaces. These antigen presenting cells (APCs) can travel through the lymphatic system to lymph nodes where they activate the adaptive immune response through stimulation of T cells.
Early in the adaptive immune response, before a targeted response can be initiated, B cells will release a first wave of generic antibodies that are designed to bind to and neutralize the virus. These first antibodies tend to be short-lived, lasting only a few weeks, and traditionally have relatively weak affinity, binding, and neutralizing abilities – but the body can produce them quickly and thus begin the process of fighting off the invading virus.
While this early stage antibody production is happening, the immune system begins creating more targeted and effective antibodies in the germinal center, a structure located in the lymph nodes and spleen. As part of this process, B cells begin to rapidly produce antibodies with random mutations to increase the likelihood that some of these will effectively recognize the virus and fight off the infection. T cells then help to select the winners (the cells producing the most promising antibodies), while killing or redirecting those cells producing less effective versions in a cycle of immunity that ultimately helps the body produce both plasma B cells (these cells generate and secrete antibodies and are highly optimized to prevent infection for years) and memory B cells (these cells remain long after infections clear and can produce more plasma cells if the infection is encountered again).
T Cells and cross-immunity between COVID-19 and SARS
A study from Duke-NUS Medical School, in collaboration with the National University of Singapore Yong Loo Lin School of Medicine, Singapore General Hospital, and National Centre for Infectious Diseases, was published in Nature in July 2020.
This research found the presence of long-term memory T cells in patients who had recovered from COVID-19, as well as in patients who had recovered from the similar disease SARS. The SARS-recovered patients, now nearly two decades post-infection, retained memory T cells specific to SARS-CoV-1, the virus responsible for SARS disease. The researchers also noted that this group of patients demonstrated a level of protection against COVID-19 due to cross-immunity from their previous infection with SARS.
The research team also looked for the presence of SARS-CoV-2 (COVID-19) specific T cells among uninfected, healthy individuals and found that half of the population tested already had SARS-CoV-2-specific T cells, which the researchers believe could be the result of previous exposure to other coronaviruses like SARS-CoV-1 or those responsible for the common cold. This finding could be good news for the quest to develop a SARS-CoV-2 vaccine, as the most effective vaccines require an enduring T and B cell response to create protective immunity. The fact that memory T and/or B cells are detected many years after infection with SARS-CoV-1 could be a promising sign for lasting immunity, though this is still an open question in SARS-CoV-2 infection that future research will look to answer.
Mild COVID-19 and T cell response
While research has shown the presence of memory T cell production that persists for years in SARS-CoV-1 infection, we are still learning how SARS-CoV-2 interacts with the immune system. One key question is whether T cell response to the virus is related to antibody response and how this relates to disease severity. An international team of researchers from the Karolinska Institutet, Cardiff University School of Medicine at the University Hospital of Wales, Technical University of Denmark, and Universidad Nacional de San Martin got to work analyzing T cell and antibody response among 200 individuals across a wide range of exposure, infection, and disease. The team mapped the immune response and found interesting correlations between antibodies, T cells, and disease severity. The results of this research were recently published in Cell.
The research determined that, as expected, SARS-CoV-2-specific antibodies and T cells were detected among all of the studied convalescent individuals, with the strongest T cell responses found among those who had recovered from severe forms of the disease. Interestingly, T cell response could be detected months after infection among exposed (but not sick) family members as well as individuals who developed very mild COVID-19 symptoms. In addition, T cells were detected in cases where sometimes antibodies were not (including among antibody-seronegative exposed family members and convalescent patients who had developed asymptomatic and mild forms of the disease). This T cell response was not replicated among healthy control donors. These findings point to a potentially interesting role for T cells in disease contraction and severity and suggest that exposure or infection could result in durable T cell responses. However, it is unclear whether memory T cell responses will be robust and able to protect against severe forms of COVID-19 in cases where circulating antibodies are undetectable.
The role of germinal centers in COVID-19 severity and immunity
Traditionally, the most targeted and efficient antibodies are produced in germinal centers. A lack of these structures means that long-term antibody immunity to various bacteria, viruses, and other pathogens can be challenging. New research from the Ragon Institute of MGH, MIT and Harvard along with Brigham and Women’s Hospital, Faculté de médecine et des sciences de la santé de l’Université de Sherbrooke et Centre de Recherche Clinique Étienne-Le Bel, and Howard Hughes Medical Institute, was published in Cell and looked at post-mortem lymph and spleen tissue to determine whether germinal centers were present.
The researchers found that some of these severe COVID-19 patients lacked germinal centers entirely. Without this germinal center activity, the immune system could potentially be compensating by leaning on other types of antibody creation. IgD–CD27– double-negative B cells, for example, were found in the studied lymph and spleen tissue. These IgD–CD27– double-negative B cells create antibody-secreting cells of a different type, although they do not lead to the development of lasting immunity. Since germinal centers facilitate selection of high affinity long lasting B cells, the lack of germinal centers could help to explain the short lived and poor-quality B cell response that is observed in a large proportion of COVID-19 cases. This finding could be bad news for developing long-term immunity, and while research is ongoing and there are no definitive answers yet, there have been a couple recent cases of reinfection that have been reported.
Where the research is headed
Because COVID-19 creates such a wide range of severity and symptom expression among different people, immune response to the disease could also occur in a way that creates wide variance between infected individuals. Researchers are looking to better understand the underlying mechanisms to help characterize the pathogenesis of the disease. For example, TNF-alpha is known to be elevated in COVID-19 patients, which could play a role in the absence of germinal centers. Still, it remains unknown whether additional factors (or other mechanisms entirely) contribute to this outcome. More research is needed to determine what this might mean for the development of memory B cells and how all of this relates to long term immunity and vaccine effectiveness.
Long term follow-up studies on COVID-19 recovered patients are underway to determine the length of time T cell immunity can be detected, and other studies are looking at a wider scope of the exposed and uninfected population to get a better grasp on whether and how T cells can protect against COVID-19 and what impact they may have on patient progression of disease. Additional research is looking at whether memory T cells in particular could lead to protective immunity, and if so, for how long any such immunity would remain. As a whole, the scientific community continues to advance our understanding of SARS-CoV-2 and COVID-19 which will undoubtedly lead to better therapeutic treatments and, with any luck, the development of effective vaccines.
T cell and B cell isolation
At Akadeum Life Sciences, we are focused on supporting research that advances human health, including the groundbreaking efforts happening worldwide to help bring about an end to the ongoing pandemic. Akadeum has developed a range of products that harness microbubbles to streamline the process of cell separation for COVID-19 and other immunological research applications.
- Our T cell isolation kits and B cell isolation kits use a simple 30-minute microbubble workflow to deliver a highly-enriched, optimized sample of healthy, happy cells that are ready for downstream processing and analysis.
- We also have an RBC Depletion kit that takes only 10 minutes and can be completed in three simple steps, delivering a fast, easy, and gentle sample prep that requires no special equipment.
- Our Streptavidin bubbles can be used for a wide range of applications, and our team is here to help you optimize your application with custom protocol development and consulting.
- Our microbubbles aren’t limited! We partner with various biotech & pharma companies and research institutions to leverage the power of our technology to solve long-standing problems in sample preparation.
We’re here to help streamline and simplify your workflow. Get in touch with our team today, we can help you determine the best product(s) to suit your application and generate a custom quote to meet your exact research need. Standing orders and bulk discounts are also available, please contact us for details.
Further Reading and References:
SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls (Nature)
Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19 (Cell)
Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19 (Cell)
Some COVID-19 Patients Lack Key Structures for Antibody Creation (The Scientist)



