T Cell Depletion: Advanced Techniques & Applications
T cells play a central role in immune function. However, in many laboratory and clinical applications, endogenous T cell depletion is essential for experimental control and therapeutic success. In cell therapy manufacturing, immunotherapy development, or transplantation research, removing endogenous T cell populations ensures optimal outcomes by reducing unwanted immune responses and improving cell culture purity.
Traditional T cell depletion techniques, such as magnetic bead-based separation, have long been used to isolate and refine cell populations. Still, these methods often introduce mechanical stress, require specialized equipment, are not scalable, and can compromise cell viability. Akadeum Life Sciences has pioneered an innovative alternative: buoyancy-activated microbubble technology, which offers a gentle, efficient, and highly scalable approach to T cell depletion in the lab.
This guide examines the scientific foundations, laboratory-based applications, and best practices for T cell depletion, while highlighting how Akadeum’s microbubble kits enhance efficiency, purity, and scalability in cell separation workflows.
Comparing T Cell Depletion Methods: Magnetic Beads vs. Microbubbles
| Feature | Magnetic Bead-Based Depletion | Akadeum Microbubble Depletion |
| Processing Time | Multi-step process requiring washes, >4 hours | Four-step process, <1 hour, no intermediate washing steps |
| Cell Viability | Mechanical stress can reduce viability | Gentle flotation-based separation preserves cell health |
| Specialized Equipment | Requires magnets, columns, and wash steps | No magnets, columns, or additional equipment |
| Scalability | Limited scalability for large-volume samples requires multiple, repeated processing steps | Compatible with manual and automated workflows. Process >50 billion cells in one hour. |
| Regulatory Compliance | Some methods lack GMP-compliant options | Available in Clinical Ready (CR) and GMP-grade versions |
The Role of T Cell Depletion in Research & Cell Therapy Manufacturing
Over the years, T cell depletion techniques have evolved to meet the growing demands of cell therapy, immunotherapy research, and transplantation studies. Magnetic bead-based separation allows researchers to deplete T cells with higher specificity. However, these methods often require specialized magnetic equipment, multiple wash steps, and mechanical manipulation that can stress cells, reduce viability, and limit scalability, especially in high-throughput workflows.
Buoyancy-based microbubble technology has emerged as a gentler and more efficient alternative for T cell depletion, enabling rapid, gentle, large-scale depletion without the need for magnets or extensive sample handling. This shift marks a critical advancement in cell separation workflows for research and cell therapy manufacturing.
Applications in Immunotherapy and Transplantation Research
T cell depletion is crucial in several cell-based research and therapeutic development areas, ensuring the production of high-quality cell populations for high-impact applications.
CAR T Cell Therapy Manufacturing
In CAR T cell therapy, removing non-transduced T cells after genetic modification is essential to improve therapy efficacy and safety, resulting in higher yields of desired therapeutic T cells. This is particularly beneficial in allogeneic CAR T therapies, where manufacturing costs are high, providing a more pure population of the engineered T cells can make therapeutics more effective from a cost and dose perspective.
Graft Engineering for Transplantation
In hematopoietic stem cell transplantation (HSCT), depleting alloreactive T cells helps reduce the risk of graft-versus-host disease (GVHD), a life-threatening immune response in transplant recipients. Selective depletion of αβ T cells while preserving beneficial γδ T cells has been shown to enhance transplant success by maintaining immune protection without increasing the risk of rejection. Advanced depletion methods ensure safer and more effective graft engineering strategies.
Autoimmune Disease Research
Autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and Type 1 diabetes, are often driven by autoreactive T cells that attack healthy tissue. In preclinical models, researchers use T cell depletion to study how removing specific pathogenic T cell populations can reduce inflammation and prevent disease progression. By refining depletion strategies, scientists are developing more targeted immunotherapies that could minimize the need for broad immunosuppressive treatments.
Drug Development & Preclinical Studies
In immuno-oncology and checkpoint inhibitor research, controlling T cell populations allows researchers to precisely assess how novel drugs interact with the immune system. T cell depletion ensures experimental consistency, making it easier to evaluate drug candidates in tumor microenvironment studies, cell therapy development, and personalized medicine approaches.
T cell depletion is not just about removing unwanted populations—it’s crucial in refining and optimizing cell-based therapies, ensuring that downstream applications yield reproducible, high-quality results.

Challenges in T Cell Depletion and How to Overcome Them
While T cell depletion is essential for many applications, researchers often encounter technical and process-related challenges that impact cell yield, purity, and workflow efficiency. Some of the most common obstacles include:
- Cell stress & viability loss. Harsh separation techniques can compromise cell health, leading to apoptosis or reduced functionality. Maintaining gentle, low-stress depletion conditions is essential for preserving cell integrity.
- Incomplete depletion. Some methods leave residual T cell populations, which can reduce therapeutic effectiveness and increase the risk of adverse events.
- Scalability issues. Traditional depletion methods may not easily scale from small research batches to large-scale cell therapy manufacturing, requiring adjustments that introduce variability.
- Regulatory compliance & reproducibility. Meeting Good Manufacturing Practice (GMP) and clinical research standards requires consistent T cell depletion with minimal batch-to-batch variability.
The Challenge of Automation & Workflow Standardization
One of the biggest challenges in large-scale T cell depletion is achieving a standardized workflow that does not compromise purity or cell health. Many traditional methods require multiple manual steps, increasing the risk of variability between runs. Additionally, column- or magnet-based methods can create bottlenecks and increase cost in high-throughput environments where processing and release testing billions of cells per batch is necessary for clinical applications.
How Akadeum’s Microbubble Technology Solves These Challenges
Akadeum’s microbubble technology overcomes these obstacles by offering a scalable, automation-friendly depletion process that eliminates excessive manual intervention, reduces batch-to-batch variability, enables more cost-effective release testing with larger batch sizes, and ensures consistent depletion efficiency. Microbubble technology removes the need for columns or magnets, enabling researchers to standardize T cell depletion protocols across research-scale experiments and GMP-compliant clinical manufacturing. This makes it easier for therapeutic developers to meet regulatory standards while maintaining high-purity, high-yield cell populations.
The Science Behind T Cell Depletion
T cell depletion is a critical step in laboratory workflows. It allows researchers to selectively remove endogenous T cell populations, refine experimental conditions, improve therapeutic cell preparations, and minimize unwanted immune system responses. The process relies on targeted separation techniques that distinguish T cells based on surface markers.
How T Cell Depletion Works in the Lab
T cell depletion typically involves negative selection methods, where unwanted T cells are labeled and removed, preserving other immune cell populations for further study. This is generally achieved using one or a combination of separation techniques.
- Antibody-based targeting. Monoclonal antibodies bind to specific T cell surface markers (e.g., CD3, CD4, CD8), enabling targeted depletion.
- Magnetic bead-based depletion. T cells bound to antibody-coated magnetic beads are removed using a magnetic field.
- Microbubble-based depletion. A newer, gentler, and more efficient approach that uses buoyancy-activated microbubbles and biotinylated antibodies to lift and remove targeted T cells without excessive mechanical stress.
By selectively removing T cells, depletion techniques ensure that downstream applications, such as stem cell transplantation, CAR T therapy development, and immune system modeling, yield purified, high-quality cell populations.
Akadeum’s Microbubble Technology: A Better Approach to T Cell Depletion
Traditional T cell depletion methods, such as magnetic bead-based separation, require specialized equipment, multiple wash steps, and complex handling that can stress cells and reduce yield. Akadeum’s microbubble technology offers a breakthrough alternative—a buoyancy-based approach that enables rapid, gentle, and scalable T cell depletion without magnets or columns.
Unlike conventional methods that rely on external forces like magnets, Akadeum’s microbubbles leverage the power of buoyancy to remove endogenous T cells from suspension gently. This results in:
- Higher cell viability. Cells remain healthier due to the rapid, low-shear, stress-free process.
- Greater recovery of valuable non-T cell populations. CD3- cells remain untouched.
- Scalability from research to clinical manufacturing. Compatible with both manual processing and high-throughput workflows using industry-standard equipment. Larger batch sizes are more readily enabled, saving time, labor, and release testing costs.
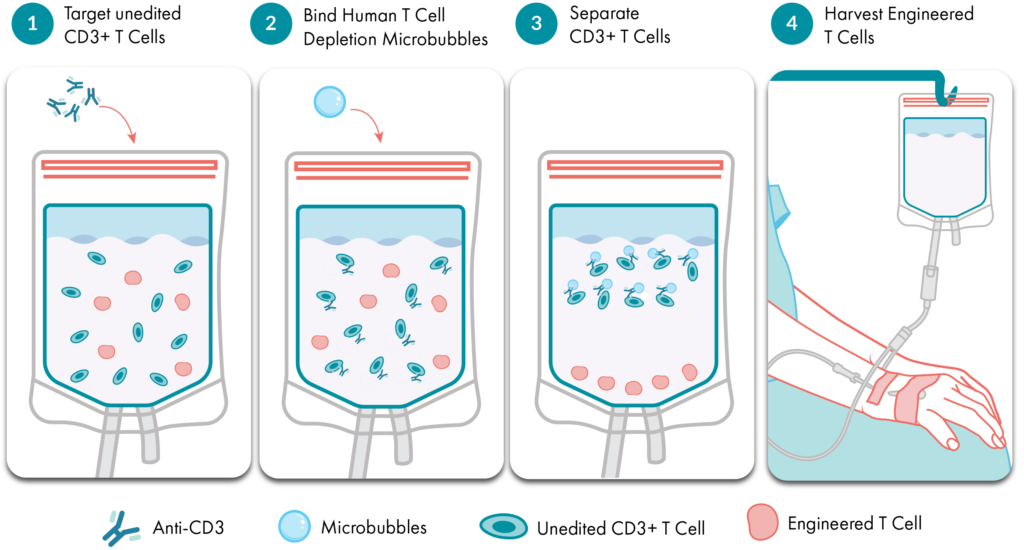
How Akadeum’s Microbubble Technology Works
Akadeum’s microbubble T Cell Depletion kits work in just four easy steps.
- Antibody targeting. Akadeum’s Human T Cell Depletion Kit uses a proprietary biotinylated antibody cocktail that binds to the CD3+ T cell receptor complex on the cell surface.
- Microbubble binding. The antibody-labeled T cells interact with Akadeum’s buoyancy-activated, streptavidin-coated microbubbles.
- Separation by buoyancy. The microbubbles lift the bound, unwanted T cells to the surface, allowing easy removal of the unwanted T cells via gentle aspiration or centrifugation. Alternatively, if using an in-bag depletion approach, the desired cells can be drained into a new vessel after separation.
- Untouched cell recovery. The remaining CD3- cells are left intact and ready for cell culture, flow cytometry, or therapeutic formulation.
This streamlined process enables billions of T cells to be depleted in under an hour, making it ideal for CAR T cell manufacturing, allogeneic cell therapies, and immunotherapy research. For more details on the protocol, download the current Human T Cell Depletion Kit – CR User Guide. User Guides can be found in the Resources tab of any Product Page.
Key Benefits for T Cell Depletion
- Flexible & scalable. Works with manual protocols for small-scale research and seamlessly scales up to billions of cells in semi-automated workflows using industry-standard equipment or Akadeum’s Alerion™ Microbubble Cell Separation System.
- Higher recovery & purity. Increased gene-edited cell recovery makes it ideal for CAR T cell development.
- Healthier cells. Unlike other techniques, microbubbles eliminate physical stress and enable limited cell handling time by faster processing, preserving the viability of cells used in immunotherapy and transplantation research.
- Regulatory-ready. Available in Clinical Ready (CR) and GMP-grade versions, tested per ISO 20399, ISO 10993, and USP <1043> for compliance with cell and gene therapy ancillary material manufacturing standards. GMP compliance per 21 CFR 820, 211, and 210.
Real-World Applications of Microbubble-Based T Cell Depletion
Akadeum’s microbubble-based T cell technology is already transforming workflows in cell therapy manufacturing, research laboratories, and biopharmaceutical companies. Depletion methods further improve allogeneic CAR T workflows by efficiently removing endogenous T cells.
Researchers in immunotherapy and transplantation benefit from microbubble technology’s ability to selectively deplete T cells while preserving other immune cell populations for downstream applications. Additionally, Akadeum’s scalable platform supports the seamless transition from feasibility studies to clinical production, enabling therapeutic developers to scale up cell manufacturing without additional expensive capital equipment investments.
Whether in preclinical research or regulated clinical environments, microbubble-based depletion is a high-efficiency, cost-effective, low-cell stress solution designed to improve and address the challenges of T cell depletion, particularly for allogeneic cell therapy manufacturing.

Best Practices for T Cell Depletion
Achieving high-purity T cell depletion requires careful protocol execution, quality control measures, and optimization of sample preparation. Whether working with small-scale research samples or billions of cells for clinical applications, following best practices ensures maximal recovery, viability, and reproducibility.
Quality Control Measures for Reliable T Cell Depletion
Consistency in depletion efficiency is key to ensuring reproducible results in research and clinical applications. Labs can improve process reliability by:
- Validating cell counts pre- and post-depletion. Use flow cytometry or a cell counting instrument to confirm the efficiency of depletion.
- Ensuring proper microbubble resuspension. Thoroughly mix the microbubbles before adding them to the sample to provide uniform separation.
- Optimizing sample handling. Use gentle pipetting techniques to maintain cell viability and prevent shear stress.
- Minimizing incubation variability. Standardize incubation times and rotation speeds to improve consistency across samples.
- Conducting depletion via a closed system approach. A closed system approach, such as in-bag depletion, minimizes risk for contamination and allows for easy integration into existing systems via standard tube welding practices.
These practices help maintain high recovery rates, reduce variability, and ensure the best possible purity in depleted samples.
Optimization Techniques for Maximum Cell Yield and Purity
Optimizing T cell depletion protocols requires careful consideration of timing and sample preparation to ensure high-purity cell populations with minimal stress.
Incubation times should be carefully controlled—longer incubations can improve depletion efficiency, but excessively long processing may lead to unintended cell loss. Finding the right balance between incubation time and depletion efficiency is key to maintaining an optimal workflow.
Sample preparation also plays a critical role in achieving consistent, high-yield results. Standardizing cell concentrations before depletion helps maintain reproducibility across different batches. Additionally, optimizing buffer composition supports cell integrity and function throughout the process.
By refining these parameters, researchers can improve the efficiency, purity, and viability of depleted samples, making microbubble-based depletion an ideal solution for preclinical research and clinical applications.
Regulatory and GMP Compliance Considerations
Akadeum’s Clinical Ready (CR) and GMP-grade kits meet stringent quality and compliance standards for researchers working in GMP-regulated environments.
- Biocompatibility testing. Clinical Ready products are tested under ISO 20399 and ISO 10993 for safety in cell and gene therapy applications for ancillary materials.
- Sterility and endotoxin testing. Manufactured under USP <71> sterility and USP <85> bacterial endotoxin standards.
- Regulatory documentation. Fully compliant with 21 CFR 820, 210, and 211 for clinical research and therapeutic development.
These regulatory assurances make Akadeum’s T Cell Depletion Kits a trusted solution for transitioning from research to clinical-scale manufacturing.
Integration into Cell Therapy Manufacturing Workflows
Microbubble-based depletion is designed for seamless integration into advanced cell therapy workflows. Labs and manufacturers can:
- Automate T cell depletion at scale. Process billions of cells using the Alerion™ Microbubble Cell Separation System or in-bag using industry-standard equipment you already own.
- Standardized depletion protocols. Ensure consistent and reproducible T cell depletion across multiple batches.
- Reduce hands-on time and GMP suite time. Microbubble-based depletion minimizes time spent processing, increasing efficiency in high-throughput settings and enabling healthier cells from minimal handling times. This also reduces the need for GMP suite time, resulting in additional cost savings.
- Reduce release testing costs. the scalability of microbubble-based depletion enables larger batch sizes, reducing release testing costs.
By integrating scalable, semi-automation-ready depletion strategies, researchers can confidently accelerate clinical translation and commercial manufacturing.
Future Innovations in T Cell Depletion
As cell therapy and immunotherapy research continue to advance, the demand for high-efficiency, scalable, and automation-ready cell separation technologies is growing. While effective, traditional T cell depletion methods face scalability, cell viability, high costs and process efficiency limitations. Innovations in buoyancy-based separation are expected to drive the next generation of cell depletion technologies, offering faster, more precise, and less labor-intensive workflows.
Microbubble-based separation has already transformed depletion at scale. Still, future developments may include enhanced antibody cocktails that improve specificity and integrated automation solutions that further reduce hands-on time and variability in clinical manufacturing.
Akadeum’s Commitment to Advancing Cell Separation Science
Akadeum Life Sciences remains at the forefront of next-generation T cell depletion, continuously refining its microbubble-based approach to meet the evolving needs of cell therapy, immunotherapy, and transplantation research. Akadeum is committed to enabling efficient, reproducible, high-purity cell separations from early research through commercial manufacturing by prioritizing cell health, automation, scalability, and regulatory compliance.
With innovations in Clinical Ready (CR) and GMP-grade solutions, Akadeum is addressing today’s challenges in T cell depletion and paving the way for future breakthroughs in precision cell separation for cell and gene therapy applications.