How to Check Cell Viability in a Sample
Determining cell viability is essential to understanding how cells behave and how they respond to analysis or therapeutic testing. To conduct accurate cell viability tests, researchers must optimize their procedures for differentiating healthy cells from cells that are dead or damaged. Akadeum’s buoyancy activated cell sorting (BACS) technology provides a gentle and efficient way to identify and remove unwanted dead or degraded cells from a sample, leaving behind healthy cells for further study or downstream research applications.

Cell Viability
Cell viability refers to the number of living cells within a population. Determining the viability of cells in a sample is critical to both understanding cell behavior and assessing efficacy throughout research and treatment development. Conducting cell viability assays allows researchers to infer the overall cell health in samples, improve experimental procedures, and determine whether cells remain healthy through therapeutic testing.
Cell Viability Percentage
Given that cell viability is the proportion of living, healthy cells compared to the total population, the cell viability percentage of a given sample can be calculated as follows:
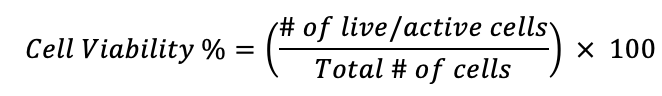
The cell viability percentage of a sample is a useful check of research efficacy as a measure of overall cell survivability. For example, calculating cell viability percentage throughout various stages of analysis informs decisions about which procedures are best suited for experimental success. Likewise, comparing cell viability percentage before and after introducing pharmacologic compounds can give insight into whether the cells-in-question will remain healthy during treatment.
How To Check Viability of Cells
Researchers employ various types of cell viability assays for cells in culture—these typically track cell proliferation, metabolic activity, or ATP content to discern cell health. Cell viability can also be inferred through first running cell toxicity assays that measure the number of dead or damaged cells in a sample and then discounting these unhealthy cells from the total population to calculate the number of viable cells.
Cell Proliferation Assays
Cell proliferation describes the ability of cells to reproduce via cell division, or cytokinesis. Since only healthy cells will divide and proliferate, while damaged, dying, or dead cells do not, researchers measure the increase in cell count in a population through various methods of cell proliferation assays in order to calculate the overall cell viability percentage of the sample.
To determine the number of cells actively dividing within a culture, cell proliferation assays typically measure DNA synthesis or overall DNA content within replicating cells through imagine, flow cytometry, immunofluorescence, or enzyme-linked immunosorbent assays (ELISA).
Cell cycle analyses are a type of proliferation assay that track the progression of healthy cells through the cell cycle. Viable cells performing proliferation first grow larger in size, then synthesize and replicate their DNA. After DNA replication, the cells grow even larger before undergoing mitosis, during which cells enclose the two replicated DNA strands with distinct nuclear membranes. Finally, two discrete cellular membranes and organelles develop around the two nuclei. To track the progression of the cell cycle, researchers commonly tag DNA with fluorescent agents—cell proliferation is then assessed based on the intensity of fluorescence observed throughout the cell cycle.
Another form of analyzing proliferation to determine cell viability is via DNA synthesis assays. As with cell cycle analyses, researchers incorporate labeling agents into newly synthesized DNA, and then measure the levels of these agents throughout the study. The presence of these labeling agents in subsequent generations of cells is correlated with the amount of DNA synthesis occurring among the cells under study, and thus is an indicator of the extent to which healthy cell division takes place in the sample population.
As viable cells undergo cytokinesis, they express high levels of specific cell cycle proteins. Detecting these proliferation proteins in a given cell culture thus provides another measure of cell proliferation within the sample. As in other forms of proliferation assays, the presence of cell cycle proteins provides an indicator of cell viability for the population in question.
As opposed to tracking the number of healthy cells performing cytokinesis in a sample, cell viability can also be determined through measuring the senescence of cells in a population. Senescence describes the degraded ability of cells to carry out cell division. Detecting markers of cell senescence such as enlarged cell size, altered gene expression, or expression of specific chemical activity allows researchers to measure the proportion of unhealthy cells in a population that are not proliferating, and subsequently determine the viability of the remaining cells in the sample.
Metabolic Activity Assays
Aside from querying actively dividing cells or discounting cells that aren’t proliferating, cell viability can also be inferred from the metabolic activity levels present in each population.
Metabolic analyses such as MTT or XTT assays first incubate a sample with a yellow-colored dye; the mitochondrial enzymes in healthy cells then convert these dyes into a purple byproduct as they produce energy to power cell activity. The number of viable cells in a population can then be determined from the amount of these byproducts within the sample by measuring the number of purple-colored cells.
Adenosine triphosphate, or ATP, is a molecule produced during the healthy cellular function that provides energy to power the activity of living cells. ATP measurement is another type of metabolic analysis that tracks the generation of ATP or the presence of byproducts of ATP consumption during normal metabolic processes.
Cell Toxicity Assays
Cell viability can also be derived from assessments of cell toxicity, which is a measure of the number of cells that die or become damaged, either during the natural course of the cell cycle ending in cell death or following treatment with pharmacological agents in the process of therapeutics development and testing.
Many approaches to counting dead or damaged cells in a population are similar to those for counting live cells in cell viability assays. One common method involves introducing a chemical marker to a sample and analyzing how the cell population responds. For example, only dead cells or cells with damaged membranes will absorb trypan blue dye—after introducing this dye to a sample, dead or damaged cells will uptake the dye and take on a blue appearance, while healthy, viable cells with intact cell membranes will remain uncolored. In this way, researchers can easily differentiate between healthy cells and dead or dying cells in order to calculate cell toxicity percentage (and thus cell viability percentage) of the population under observation.
To derive cell viability percentage after conducting a cell toxicity assay (in which dead or damaged cells are counted), first, calculate the cell toxicity percentage of the sample:
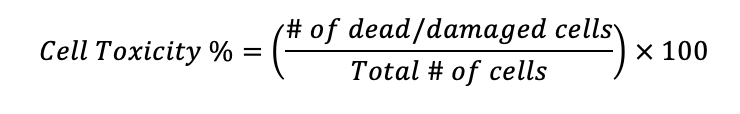
Cell viability of the sample is then found by subtracting the proportion of dead or damaged cells from the overall population count. The remaining cells can be inferred to be viable:
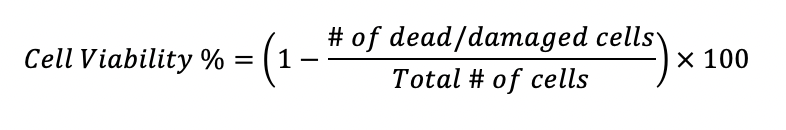
Improving Cell Viability with BACS
Degradation of cells in a population can occur due to natural cell death, in response to therapeutic testing, or as a result of the experimental procedure. Whatever the cause, when cells become dead or damaged, they leave behind cellular debris—both unhealthy cells and the debris they produce can impede the accuracy of cell viability assays if they are miscounted as whole cells or are unable to be differentiated from healthy cells in the sample.
To determine how a particular process or treatment will affect healthy cells in the body, the sample population being tested must be comprised of viable cells. As such, being able to isolate healthy cells from cellular debris or dead cells within a sample is crucial to being able to accurately determine cell viability and the efficacy of therapeutic testing.
Akadeum Life Sciences has developed a breakthrough technology for separating healthy cells from dead or damaged cells that is affordable, easy to perform, and yields viable cell populations with high purity. Akadeum’s Buoyancy Activated Cell Sorting (BACS) microbubbles are gently mixed into a sample. The microbubbles are coated with application-specific affinity molecules that bind to the surface of target cells or cellular fragments before lifting their targets to the surface of the sample for collection or removal.
Dead cell removal and general cell separation with BACS are as effective as traditional methods such as Fluorescence Activated Cell Sorting or Magnetic Activated Cell Sorting, while imposing far less risk to cellular health from exposure to harsh chemicals or enzymes, shear forces, or powerful magnetic gradients. Moreover, BACS with Akadeum’s cell isolation kits requires no additional equipment or specialized training and is suited for processing large volumes of samples at scale and speed.
To learn more about the importance of cell viability and how Akadeum’s Buoyancy Activated Cell Sorting technology is revolutionizing cell separation and cell viability assays, you can see a 3-minute recap of our recent webinar on dead cell removal with microbubbles, or explore our full webinar archive.


