Akadeum Microbubbles and G-Rex® Bioreactors: an Instrument Free Cell Therapy Workflow
Wilson Wolf G-Rex® Bioreactors are widely used in CAR T and adoptive cell therapy workflows due to their scalability, gas-permeable design, and ability to support high-density T cell expansion. However, even with an efficient culturing platform, many workflows still rely on instrument-based or magnetic isolation systems, which complicate setup, increase costs, and risk compromising cell health.
Akadeum Life Sciences offers a more innovative alternative. With the Human T Cell Leukopak Isolation (TCI) Kit – GMP, users can isolate more T cells, leveraging the gentleness of flotation upstream of using G-Rex® to culture—all in a closed, instrument-free format. This integration is readily compatible with tube-welding systems already in the lab, reduces handling steps, and supports the development of healthier, more functional T cells.
Whether you’re working in a clinical manufacturing suite or process development, combining Akadeum’s microbubble technology with the G-Rex® bioreactor platform enhances upstream cell quality while preserving the simplicity of your existing workflow and enabling an instrument-free workflow.
The Case for Simpler Cell Therapy Workflows
As cell therapy scales up, so do the demands on process design. Traditional magnetic bead-based systems for T cell isolation add complexity, cost, and risk to early-stage manufacturing. These systems:
- Require dedicated instruments and consumables
- Can be difficult to scale up to meet manufacturing needs
- Are time-consuming to run
- Risk over-activating T cells, increasing exhaustion markers like PD-1
For G-Rex® users who already rely on the platform’s efficient, large-volume culture environment, the last thing needed is an upstream method that adds bottlenecks and risks expansion success.
Akadeum’s microbubble-based T Cell Leukopak Isolation Kit-GMP changes that. It offers a gentle, efficient, and fully closed method for isolating T cells before they enter the G-Rex® bioreactor. The entire process—from antibody labeling to microbubble enrichment—can be done in standard cell processing bags and transferred via simple tube weld, maintaining sterility and minimizing hands-on time. There’s no need for open centrifugation steps or complex equipment. By simplifying the upstream phase, teams can focus on optimizing expansion without compromising the quality of their starting T cells.
Why Microbubble-Based T Cell Isolation Outperforms Magnetic Methods
Akadeum’s Human T Cell Leukopak Isolation Kit – GMP provides a high yield of highly pure T cells in under an hour, eliminating the need for magnetic beads or dedicated cell separation instruments. This negative selection approach enables gentle enrichment, supporting cell health and functionality throughout the expansion process.
In a study using Wilson Wolf G-Rex® 6M Well Plates (substitute for G-Rex® 10M-CS to maintain a closed system), Akadeum’s microbubble-based T Cell Leukopak Isolation Kit – GMP delivered clear performance advantages:
- Robust expansion: T cells isolated with microbubbles rapidly expand in the G-Rex 6M well plates
- Healthier phenotype. The microbubble approach maintains T cell memory plasticity phenotypes, hallmarks of more therapeutically relevant cells.
These benefits contribute directly to better transduction efficiency, downstream functionality, and therapeutic viability, making microbubble-enabled T Cell Isolation a meaningful upgrade.
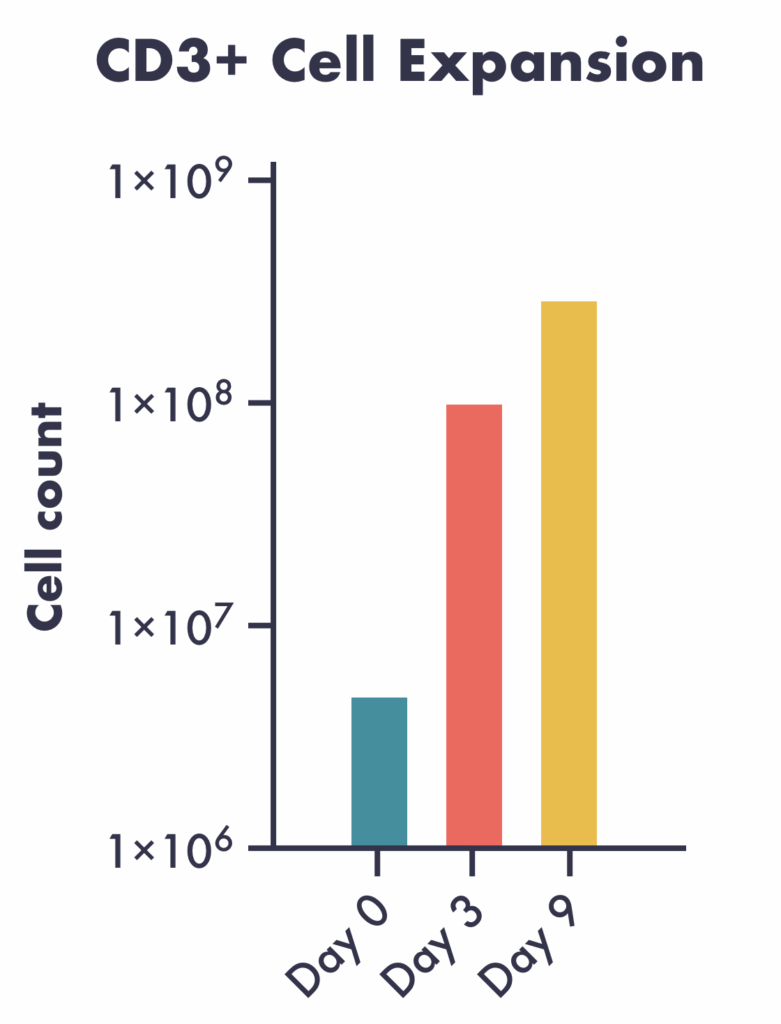
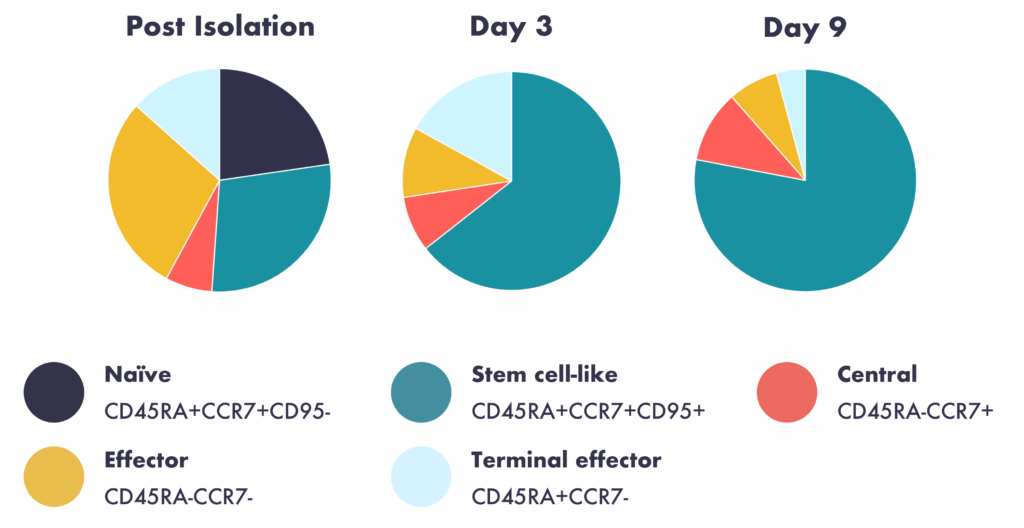
Rapid expansion post-isolation in G-Rex® 6M plates. T cell memory plasticity phenotypes are maintained at Days 3 and 9 post-seeding in the plates. These specific phenotypes are important to therapeutically relevant cells, potentially enabling more effective therapeutics.
How to Integrate Akadeum’s Kit with the G-Rex® Platform
Integrating Akadeum’s negative selection T Cell Leukopak Isolation Kit-GMP into a G-Rex®-based workflow is simple and designed with closed-system compatibility in mind. The process requires no additional instruments and fits easily into existing GMP or research workflows.
Step-by-step workflow:
- Label apheresis material with the T Cell Leukopak Isolation Kit-GMP antibody cocktail. Mix end-over-end for 15 minutes to bind unwanted, non-CD3+ cells via negative selection
- Add Akadeum microbubbles to the cell transfer bag. Continue end-over-end mixing for another 15 minutes to bind and buoyantly label the previously tagged cells, gently floating them to the top of the transfer bag within 15 minutes.
- Tube weld to G-Rex® culture vessel for downstream processing. The T cells can be transferred via sterile weld to a G-Rex® 10M-CS or larger system, maintaining a closed workflow from start to finish.
This integrated process provides a comprehensive, instrument-free solution for cell isolation, thereby reducing costs, simplifying logistics, and maintaining the quality of your therapeutic cells.
FAQs
How do microbubbles impact T cell phenotype?
Unlike traditional magnetic methods, Akadeum’s microbubble-based isolation uses a rapid, flotation-based negative selection approach, leaving T cells completely untouched. This results in improved cell health and viability from reduced cell handling and cell manipulations.
Can this workflow remain fully closed?
Yes. All steps—from selection to transfer to expansion—can be performed in closed, sterile bags and G-Rex® bioreactors using standard tube welding processes and equipment. Is the T Cell Leukopak Isolation Kit available in GMP format?
Absolutely. The Human T Cell Leukopak Isolation Kit is available in research-use-only (RUO), GMP-grade, and Clinical Ready formats. It is suitable for early process development (RUO, GMP) and clinical-scale manufacturing (Clinical Ready).
How does the integration compare to magnetic workflows?
Akadeum’s kit does not require cell separation instrumentation – simply replace magnetics with microbubbles for apheresis cell isolation, then go through your standard cell therapy workflow downstream of isolation. Studies combining Akadeum’s in-bag workflow and G-Rex® systems have shown comparable or superior performance, making it a compelling alternative to magnetic bead methods with the added benefit of time and cost savings. Should you have any questions, Akadeum’s technical team is composed of the world’s experts in cell separation. We are here to walk side by side with you in your integration journey.
Upgrade Your CAR T Cell Therapy Without Adding Equipment
Akadeum’s microbubble-based T Cell Leukopak Isolation Kit – GMP, combined with the G-Rex® platform, facilitates closed-system, instrument-free isolation and expansion. Ready for therapeutic applications, Akadeum’s kit reduces cell handling time, leaves cells untouched with a negative selection approach, and avoids the downstream complications of bead removal.
Simplify your workflow and eliminate reliance on magnetic particles and specialized cell separation equipment. Akadeum is here to help you maintain control, reduce failure risk, reduce processing steps, and scale up and out with confidence.
Ready to enhance your CAR T cell workflow?
- Download the application note to see performance data.
- Contact Akadeum to request a protocol or sign up for the Evaluation Program.
- Explore the Human T Cell Leukopak Isolation Kit – GMP to get started.
Better inputs lead to better therapies—start upstream with Akadeum.