Posters
Rethinking Cell Separation for Future Successes: GMP Microbubbles for Next-Generation Cell Therapy Workflows
Published on Oct 1, 2025 Share
Microbubbles are an effective solution to cell separation challenges in cell therapy. This data demonstrates the various ways that microbubbles are being deployed in cell therapy workflows—resulting in healthier cells, higher yields, and greater potency and persistence. This poster was presented at the recent CAR-TCR Summit, the world’s leading conference dedicated to the end-to-end coverage of every stage of cell therapy development.
Healthier Cells with Akadeum’s Microbubbles: A Novel Approach to Gentle and Efficient Cell Isolation
Updated on Sep 30, 2025 | Published on Jun 3, 2025 Share
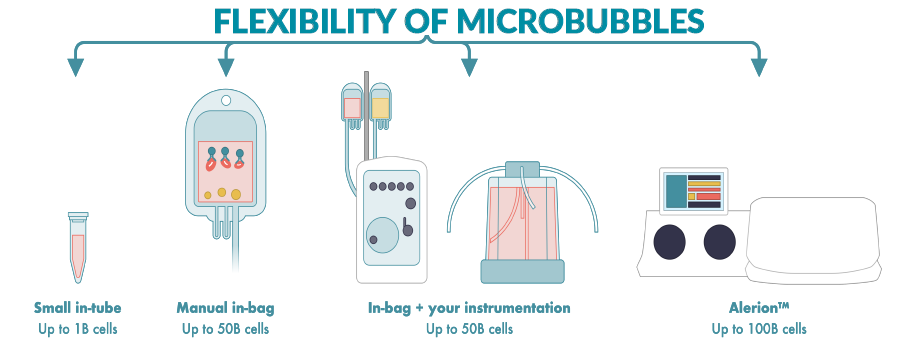
Akadeum’s microbubbles offer a simple yet powerful shift in cell separation – negative selection that can be incorporated directly into existing workflows. On this poster, which was presented at ASGCT 2025, you see the data from instrument-free processing, integration of microbubbles into existing equipment, along with examples of various starting sample materials, compatible protocols and workflow.
Evaluating Buoyancy Activated Cell Sorting for T Cell Isolation in CAR-T Manufacturing
Updated on Jun 7, 2025 | Published on Jun 2, 2025 Share
As the CAR T and TCR landscape evolves, robust manufacturing solutions that enable increased access to these transformative therapies through reduced cost and complexity must be developed. An alternative cell separation strategy that may improve manufacturability of these therapies is the isolation of T cells by negative selection using BACS. ElevateBio’s ISCT 2025 poster evaluates the feasibility of Akadeum’s BACS technology and characterizes the performance of T cells isolated using …
End-to-End Production of T Cells on the Cocoon Platform, using BACS for Direct Isolation
Updated on Jun 7, 2025 | Published on Jun 2, 2025 Share
In this work we present an automated 9-day T cell isolation and manufacturing process on the Lonza Cocoon®️ Platform using the Akadeum®️ Human T Cell Leukopak Isolation Kit – GMP Grade, providing high purity, recovery, and yielding a T cell product with desirable phenotypes for CAR-T therapy applications.