Akadeum's microbubble technology improves production and engineering efficiency of therapeutic CAR-T cells in a scalable manner.
View Our ProductsOutcomes Matter: Microbubbles outperform other cell separation methods:
- Capacity of 100+ billion cells
- Use with raw, platelet-washed, or cryopreserved material
- Negative selection capability
- Compatible with instrumentation in your lab
- Simple, easy-to-use workflow
Everything cell therapy scientists are looking for in cell separation technology.
Superior Cell Health
Higher Potency
Greater Persistence
80% Time Savings
Smaller Footprint
Regulatory Ready
Seamless Integration. Scalable by Design. Validated Performance.
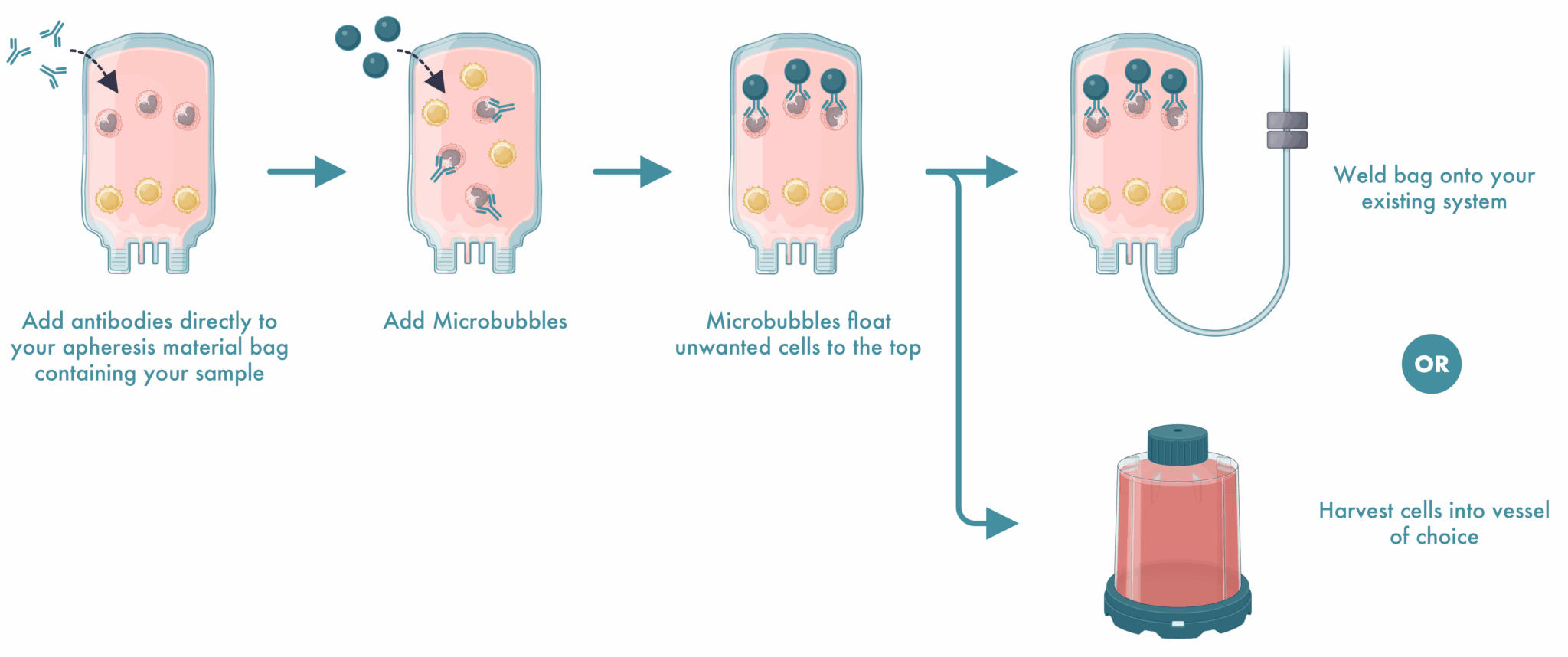
What Our Customers Are Saying
With the microbubble leukopak isolation kits, we have been able to isolate more cells in a way that’s faster and easier than ever before. ZenBio (a BioIVT company) prides itself on providing the best quality products and solutions for our research partners and tools like Akadeum’s microbubble isolation kits help us deliver on our mission.
Will Plentl, Chief Operating Officer
ZenBio Inc. (a BioIVT Company)
We found the system easy to operate and were even able to process multiple donors on the Alerion™ simultaneously during our runs, allowing us to increase our experimental throughput, which was an additional benefit.
Julian Kopelove, Senior Process Development Associate
Poseida Therapeutics
At Comprehensive Cell Solutions, we are dedicated to streamlining our manufacturing processes, reducing turnaround times, and producing safer, more effective products. Using the Alerion™ system, we’ve achieved remarkable improvements in cell viability, purity, and recovery, while significantly reducing impurities compared to conventional magnetic separation methods. This improvement led to a higher quality intermediate which we expect would result in superior product quality downstream. Our team found Alerion™ easy to use and effective at speeding up process times.
Rubina Pal, Ph.D., Sr Director, Process & Analytical Development
Comprehensive Cell Solutions
Simplify and Unify Your Manufacturing Process
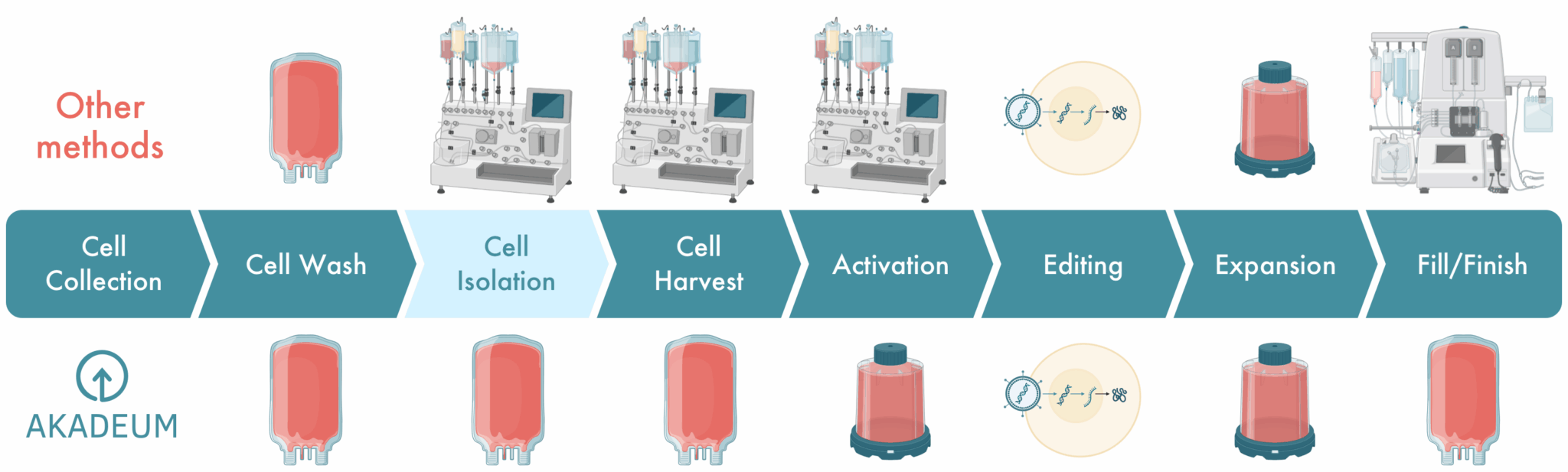
Products for Autologous and Allogeneic Workflows
Continually updated with new microbubble products